Developing Novel Protein Degradation
Inhibitors for the Treatment of Cancer

Cleave Biosciences
has a pipeline of novel, first-in-class therapeutics targeting protein degradation pathways for difficult to treat cancers as well as a deep understanding of the biology of these pathways. Our lead drug candidate CB-5083 began clinical trials in patients with Multiple Myeloma and Advanced Solid Tumors. Other Cleave programs focused on AAA ATPases and novel zinc-dependent JAMM metalloproteases are at the discovery stage.
Cleave Biosciences is focused on mining novel targets in cellular protein homeostasis pathways utilized by cancer cells for their growth and survival.

P97
P97 is an important cellular AAA ATPase also known as VCP (Valosin Containing Protein) that has a well described role as a master regulator in the Ubiquitin Proteasome System. P97/VCP extracts misfolded proteins from membranes, such as the endoplasmic reticulum, and chaperones them to the proteasome for degradation.1 Emerging evidence suggests that cancer cells become over-dependent on protein homeostasis systems and therefore inhibitors of p97 are expected to have meaningful anti-cancer activity.2 Genetic knockdown of p97 leads to cell death in a number of solid tumor cell lines and compounds that inhibit p97 have been discovered which lead to cancer cell death in various pre-clinical models.
Cleave has synthesized more than 500 p97 inhibitors and we have selected CB-5083 as a drug candidate which has begun testing in Phase 1 clinical trials.
CB-5083
CB-5083 recently began Phase 1 testing in relapsed/refractory or refractory multiple myeloma, and advanced solid tumors. CB-5083 is a potent, highly selective, oral p97 inhibitor with broad in vivo activity in both multiple myeloma and solid tumor xenografts. CB-5083 is a potent inhibitor of endoplasmic reticulum associated degradation and induces a lethal unfodled protein response. Multiple myeloma is a cancer with a well-known dependence on protein homeostasis for survival with the proteasome inhibitors bortezomib and carfilzomib as FDA approved drugs. These agents are not effective in solid tumors owing to both chemistry and pharmacology limitations. Not only has Cleave demonstrated activity in solid tumors with CB-5083, we have also identified molecular features that correlate to increased anti-tumor activity in preclinical models.
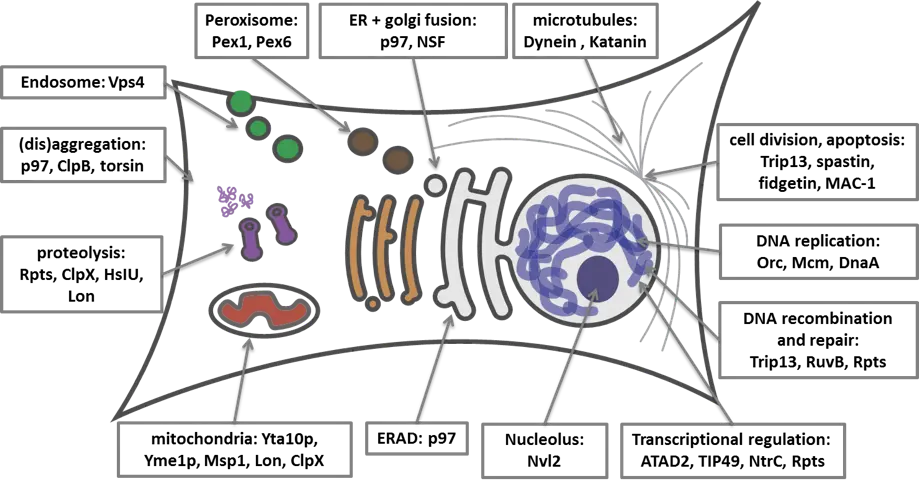
The AAA (ATPases Associated with diverse cellular Activities) ATPase family of enzymes is involved in a wide variety of fundamental cellular processes including protein homeostasis, transcriptional and translational control, DNA replication, membrane fusion, cell division and apoptosis. AAA ATPases are mechano-enzymes that use the energy derived from ATP hydrolysis to reshape protein/protein, protein/membrane and protein/DNA/RNA complexes. Several AAA ATPases are known to play important roles in cancer biology and present the opportunity to discover novel drugs that will have a differentiated mechanism of action compared to other commonly targeted protein classes such as protein kinases. Given the success of developing a small molecule inhibitor to p97 (CB-5083), Cleave Biosciences is currently conducting drug discovery efforts targeting several AAA proteins as oncology targets. Our aim is to build a pipeline of clinical candidate molecules for novel AAA targets.
Protein study techniques
Western Blot
is a widely used analytical technique for detecting and quantifying specific proteins in a sample, making it a cornerstone in protein research. The process begins with the separation of proteins by SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis), which resolves proteins based on their molecular weight. After electrophoresis, the proteins are transferred onto a membrane, typically made of nitrocellulose or polyvinylidene fluoride (PVDF), in a process known as blotting. This membrane provides a stable surface for subsequent probing. The target protein is identified using a primary antibody specific to the protein of interest, followed by a secondary antibody conjugated to a detection system, such as an enzyme (e.g., HRP or alkaline phosphatase) or a fluorescent label. Detection is achieved using chemiluminescent, chromogenic, or fluorescent methods, producing a signal that correlates with the amount of protein present. Western Blot is particularly valuable for confirming the expression of proteins, studying post-translational modifications, and validating results from other proteomic analyses. Its specificity and versatility make it indispensable in fields like molecular biology, immunology, and biochemistry, although it requires careful optimization to avoid nonspecific binding and ensure reproducibility.
ELISA
Enzyme-Linked Immunosorbent Assay is a highly sensitive and specific technique used to detect and quantify proteins, such as antibodies, antigens, cytokines, and enzymes, in complex biological samples. The assay relies on the interaction between an antigen and its corresponding antibody, coupled with an enzyme-linked detection system. There are four main types of ELISA: direct, indirect, sandwich, and competitive, each tailored to specific experimental needs. In a typical sandwich ELISA, a capture antibody is immobilized on a solid surface, such as a 96-well plate, to bind the target protein from the sample. After washing away unbound substances, a detection antibody, which is linked to an enzyme (e.g., horseradish peroxidase or alkaline phosphatase), is added. Upon addition of a substrate, the enzyme catalyzes a colorimetric, fluorescent, or chemiluminescent reaction, producing a measurable signal proportional to the amount of target protein. ELISA is widely used in protein research, diagnostics, and drug development due to its high throughput, quantification accuracy, and compatibility with diverse sample types, including serum, plasma, and cell culture supernatants. Proper optimization of antibody specificity, incubation conditions, and detection reagents is crucial for reliable and reproducible results.